The Deathstalker scorpion is about the size of your palm. It’s yellow and surly, its venom a seething cocktail of neurotoxins.

And somewhere in that poison soup is a very special little molecule, called chlorotoxin, designed to penetrate a prey animal’s brain. That effect happens to come in very handy: while it’s in there, it sticks to cancer cells while slipping right by healthy ones.
Jim Olson, a pediatric oncologist at Seattle Children’s Hospital and a researcher at the Fred Hutchinson Cancer Research Center, put that toxin to work.
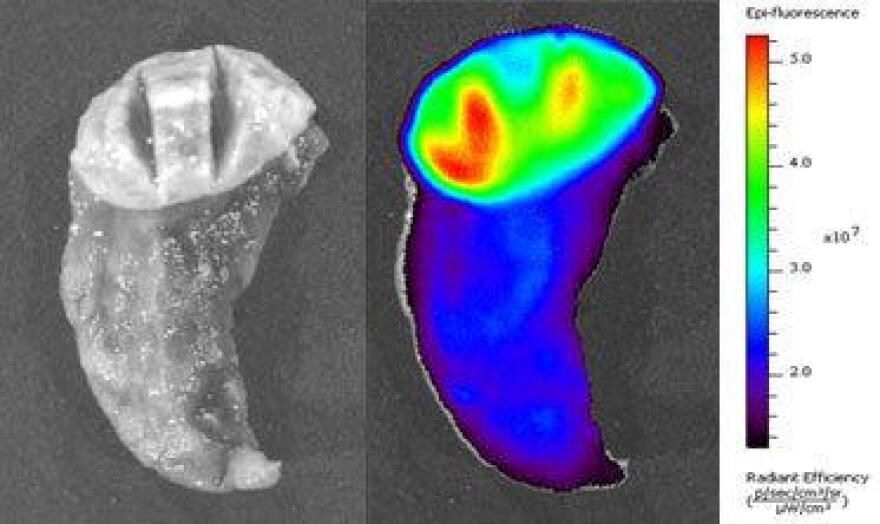
“We attached a little a molecular flashlight to that,” Olson said in a promotional video. “And so when we put it into the bloodstream, the scorpion toxin finds the cancer cells, and drags the flashlight into them and makes them glow brilliant.”
Imagine for a second that you have to cut into someone’s brain. This drug, called Tumor Paint, may be able to show the surgeon a literal shining line between the cancer cells and the precious, healthy brain tissue.
It could be a major breakthrough if it proves out. But developing Tumor Paint convinced the researchers they could be on to something even bigger: a whole new class of drugs.
Drugs In Nature

Chris Mehlin, one of Olson’s collaborators at Fred Hutch, said these little proteins, called knotted peptides or knottins, occur all over in nature.
“Horseshoe crab peptides, spider peptides, these are made in sunflowers, in petunias — really, lots of situations in nature where one organism is trying to influence another, if you can call, like, getting stung by a scorpion ‘influencing,’” Mehlin said.
Those peptides have qualities most pharmaceuticals don’t, which has researchers hoping they could lead to therapies for a whole range of diseases, from cancer to Alzheimer’s.

Most drugs, whether it’s aspirin or Viagra, interrupt molecular machines by throwing in a teeny-tiny monkey wrench. But in some cases, the moving parts you’re trying to jam are too big for that to work. So you need something bigger, but not so big that it can’t fit through cell walls, or through the blood-brain barrier. It has to be something in the middle, something durable, something like a knotted peptide.
“So we figure these are a little bigger than the small molecules, [but] should still be able to get into cells. So we’re in sort of a sweet spot,” Mehlin said.
Microscopic Origami

So why aren’t the drug companies doing this already, making billions and curing cancer?
These small proteins turn out to be really hard to make. They don’t just have to have the right building blocks in the right order, they’re also precisely folded like microscopic origami. In order to function, they have to be folded just right. That’s where Olson’s team had its other breakthrough.
“My team has learned more in the last 11 months than the top 20 pharmaceutical companies put together in the last 20 years around these mid-size drugs,” Olson said. “And we’ve gone from the ability to make 12 of these a year to being able to make 10,000 in three weeks.”
Cells On The Factory Floor

The secret sauce can be found in a tray of gyrating flasks in the team’s lab at Fred Hutch. Each contains a solution full of mammalian cells, specially programmed to excrete the desired protein. The trick turned out to be not trying to do the complicated folding yourself, but to reprogram a cell already expert at assembling proteins to do it for you.
“We have three big incubators here that look like big pizza ovens,” said project co-director Colin Correnti. “And we can literally scale this thing up to 20 liters of cell culture producing any single knottin. And that would actually provide us with enough material to do a whole plethora of studies.”
What they are mass producing in here are not thousands of new drugs; they are drug candidates. The team must comb through them for the few promising ones and throw the rest away. Then they make a few thousand variations on that handful, winnow down again, and again and again until they have a drug.
'This Is Overly Ambitious'
Each step takes money. But since the work remains largely unproven, and doesn’t fit well into the conventional process for drug discovery, Olson said it’s been hard to get funded.
“It’s very difficult to get grant support for the most innovative work. In many cases, we got back grant reviews that said this is overly ambitious, or you may not succeed in the time frame of the grant,” Olson said.
But his team is taking a longer view: if they do succeed, it opens up a whole unexplored landscape for treating diseases. They’ve already overcome a major obstacle in making their drug candidates. Now, how to tackle that little matter of needing millions of dollars a year to see if it all pays off?
The answer they settled on takes the project from the realm of the unusual into truly uncharted territory.
---
Editor's Note: This is the first installment of a two-part series. Learn how Olson's team cracked their funding problem in Part 2, which will run Wednesday.








